Electrolysis is the chemical process of using an electrical current to stimulate non-spontaneous reactions. Electrolysis of water is the process of using electricity to decompose water into oxygen and hydrogen gas.
 Electrolysis Process Useful For Education In Schools Vector Illustration Canstock
Electrolysis Process Useful For Education In Schools Vector Illustration Canstock
Ionic compounds contain charged particles called ions.
What is the process of electrolysis. Hydrogen gas released in this way can be used as hydrogen fuel or remixed with the oxygen to create oxyhydrogen gas which is used in welding and other applications. What is an Electrolysis Process. For example Sodium Chloride contains positively charged Sodium ions and negatively charged chlorine ions.
Connect one end each of the two wires to the positive and negative terminals of the cell. The first electrolysis was carried out by Sir Humphrey Davey in the year 1808. This is what happens during electrolysis.
This chemical change is the one in which the substance loses or gains an electron which means it undergoes oxidation or reduction. Electrolysis is a electrochemical redox reaction brought about by the application of a direct current. The process is carried out in an electrolytic cell an apparatus consisting of positive and negative electrodes held apart and dipped into a.
Electric current is passed through the ores electrolytesolution to result in a chemical change. Fill the tumbler with tap water and dissolve common salt in it. Gold CIP Gold Processing.
Negatively charged ions move to the positive electrode during electrolysis. This experiment revealed new understandings about the way certain elements behave and how they are different from compounds and ions. Electrolysis is the process of separating or extracting the metal from the ore.
They receive electrons and are reduced. Electrolysis process by which electric current is passed through a substance to effect a chemical change. Positively charged ions move to the negative electrode during electrolysis.
It has become the consensus of expert opinion that membrane electrolysis will be the predominant process for Chlor alkali production in the future. The fundamental process of electrolysis is the interchanging of ions and atoms by the addition or removal of electrons from the external circuit. After the carbons loaded with gold are desorbed the pregnant solution is processed via ionization.
Electric current is passed through the ores electrolytesolution to result in a chemical change. A non-spontaneous reaction is one that needs energy to work while it proceeds. Dip the two free ends of the wires into the water.
Simply explained the process of electrolysis refers to decomposition of a given element under the influence of an electric current. Electrolysis is a process by which the electric current is passed through a substance which effects sort of a chemical change. The chemical change is one in which the substance loses or gains an electron oxidation or reduction.
Desorption electrolysis system process In gold electrowinning process when the electrolysis equipment is added with anions which are more easily absorbed by activated carbons AuCN2ˉ is replaced by anion and the gold desorption is achieved. Take the two wires and remove the jacket from each end using a blade. Membrane process The electrolysis using metal anodes and cathodes partitioned by Cation Exchange Membranes The membrane process has indeed passed through various stages of design and development.
Sometimes called water splitting electrolysis requires a minimum potential difference of 123. In chemistry and manufacturing the electrolysis process is a cool technique that uses a direct electric current along with positively and negatively charged metal plates to drive an otherwise non-spontaneous chemical reaction.
 What Is Electrolysis Definition Process Facts Video Lesson Transcript Study Com
What Is Electrolysis Definition Process Facts Video Lesson Transcript Study Com
 Electrolytic Process An Overview Sciencedirect Topics
Electrolytic Process An Overview Sciencedirect Topics
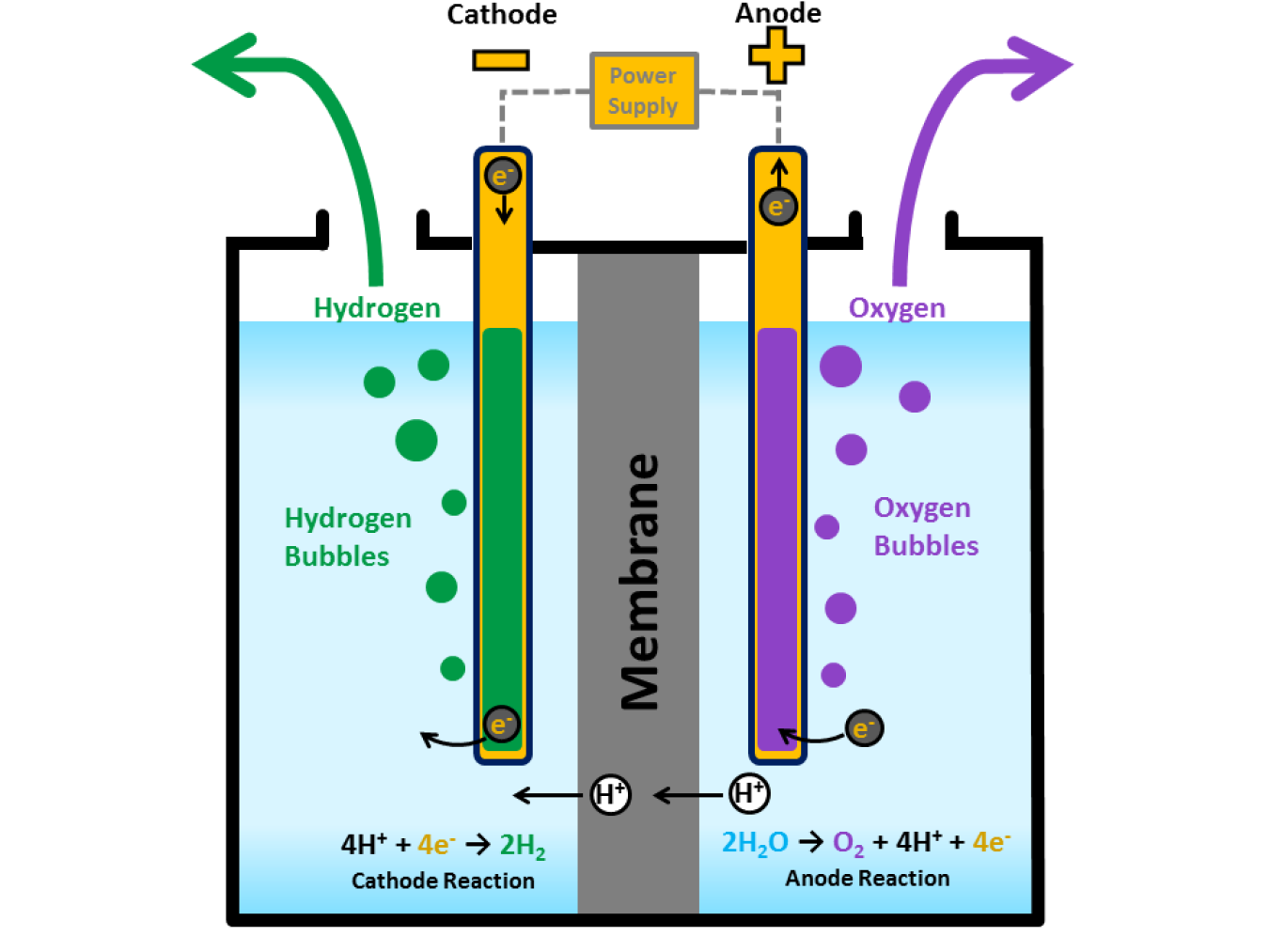 Hydrogen Production Electrolysis Department Of Energy
Hydrogen Production Electrolysis Department Of Energy
 Define Electrolysis With Example Brainly In
Define Electrolysis With Example Brainly In
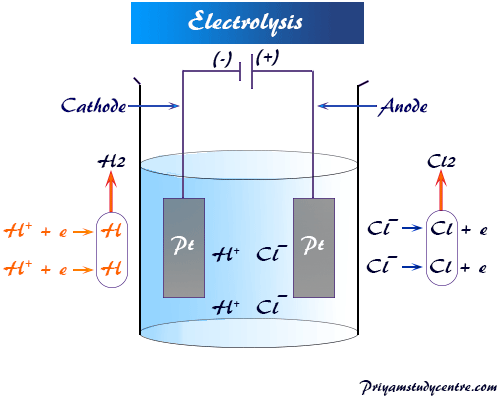
 Electrolysis Definition Formula Applications Priyamstudycentre
Electrolysis Definition Formula Applications Priyamstudycentre
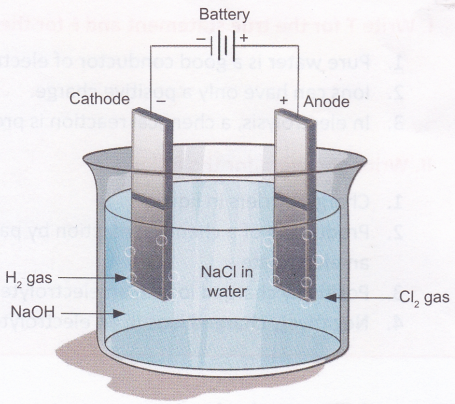 What Is The Process Of Electrolysis A Plus Topper
What Is The Process Of Electrolysis A Plus Topper
Electrolysis Of Water Wikipedia
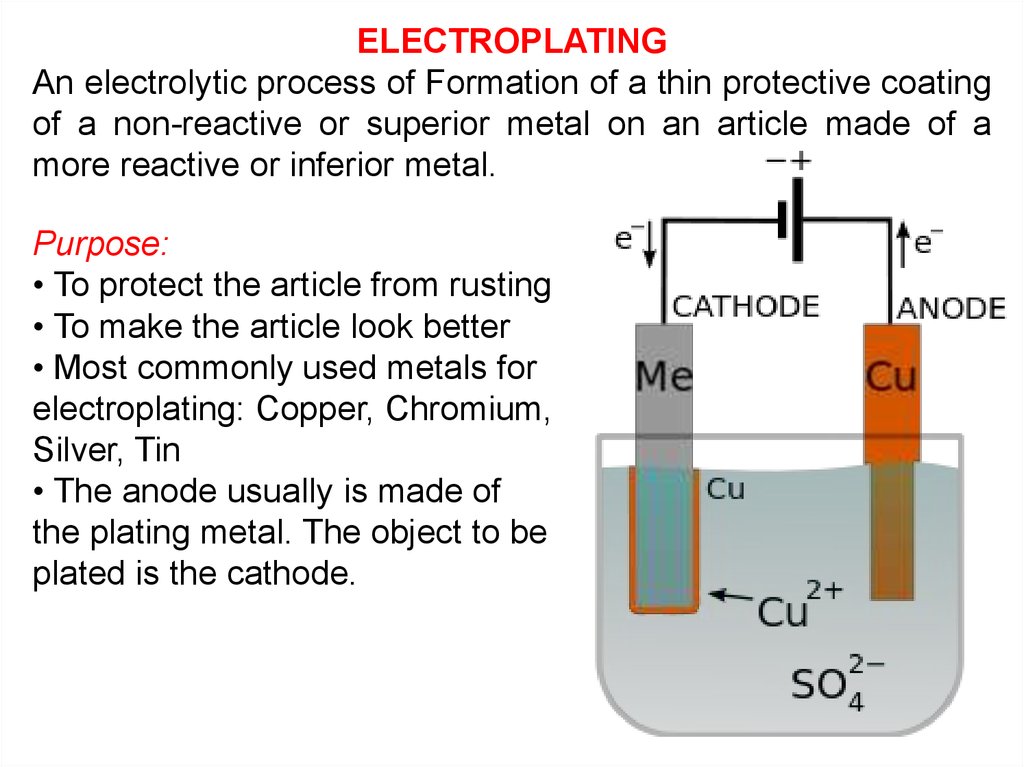 Electrolysis Online Presentation
Electrolysis Online Presentation
 The Fundamental Of Water Electrolysis Process Download Scientific Diagram
The Fundamental Of Water Electrolysis Process Download Scientific Diagram