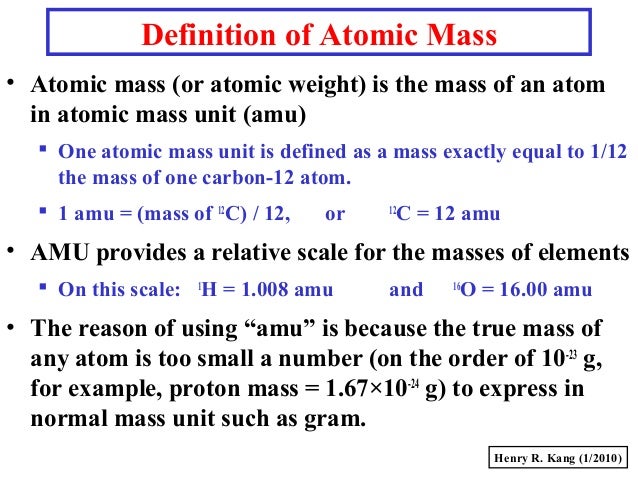
They are the smallest components of a chemical element which is a substance that cannot be broken down into simpler material. Definition of atomic mass unit.
 What Is Unified Atomic Mass Unit Determine The Equivalent Energy Of 1 U In Mev Unit Youtube
What Is Unified Atomic Mass Unit Determine The Equivalent Energy Of 1 U In Mev Unit Youtube
A carbon-12 atom is defined as having a mass of 12 u.
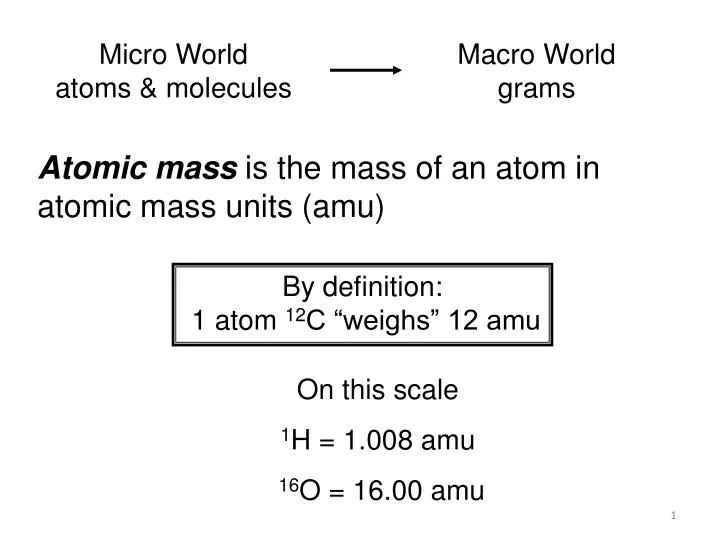
What is the definition of atomic mass unit. It is written as u. Definition of Atomic Mass Atoms are the basic units of matter. The atomic unit mass is symbolized as AMU or amu.
Atomic Mass or Weight Definition Atomic mass which is also known as atomic weight is the average mass of atoms of an element calculated using the relative abundance of isotopes in a naturally occurring element. An atom of U-235 for example. The carbon-12 atom has six neutrons and six protons in its nucleus.
Measured in terms of the atomic mass unit which is defined to be 112 of the mass of an atom of carbon-12 or 1660538921 10 24 gram. Atomic mass unit definition a unit of mass equal to 112 00833 the mass of the carbon-12 atom and used to express the mass of atomic and subatomic particles. An atomic unit of mass is defined as accurately 112 the mass of a carbon-12 atom.
What is an atomic mass unit The atomic mass unit is a unit of measurement of weight An atomic mass unit u is a unit of mass used to express atomic and molecular weights. A hydrogen atom has a mass of 1 atomic mass unit since its mass is 1 12 the mass of carbon 12. The mass of an atom consists of the mass of the nucleus plus that of the electrons so the atomic mass unit is.
Also known as a dalton the atomic mass unit is a universally-applied measurement based on 112 the total mass of a single carbon -12 atom. Units of measure have been defined for mass and energy on the atomic scale to make measurements more convenient to express. Although the SI unit of mass is kilogram the atomic mass is often expressed in the non-SI unit dalton where 1 dalton is defined as 112 of the mass of a single carbon-12 atom at rest.
əˌtɑːmɪk ˈmæs ˌjuːnɪt abbreviation u a unit used for measuring atomic mass equal to one twelfth of the mass of an atom of carbon in its commonest form carbon-12 SMART Vocabulary. Also called dalton. Mass unit equal to exactly one-twelfth the mass of one atom of carbon-12 is called one atomic mass unit.
Atomic mass unit A unit in mass spectrometry that represents the relative scale in which the mass of 1H is given an integral value of 1 12C is 12 and so on. One atomic mass unit 1u is defined as one twelfeth 112 of the mass of an atom of carbon-12. One atomic mass unit is equal to 166 x 10 -24 grams.
The American Heritage Student Science Dictionary Second Edition. The largest known protein titin has a mass of 3 x 10 6 Da. A unit of mass equal to 1 12 the mass of an atom of the most common isotope of carbon carbon 12 which is assigned a mass of 12.
Each unique atom has a unique atomic number and atomic mass. Since 1961 by definition the unified atomic mass unit is equal to one-twelfth of the mass of the nucleus of a carbon-12 atom. The atomic number is the number of protons.
The atomic mass unit is the system of measurement designed to identify each individual unit of mass in atoms and molecules. Examples of Values Expressed in Atomic Mass Units A hydrogen-1 atom has a mass of 1007 u or Da or amu. The amu is an older unit and has been replaced by the unified atomic mass unit.
Atomic mass unit amu synonyms Atomic mass unit amu pronunciation Atomic mass unit amu translation English dictionary definition of Atomic mass unit amu. The atomic mass is the mass of an atom. A single water molecule is made up of two hydrogen atoms and one atom of oxygen.
A very very small unit of mass used to express the mass of atoms and molecules conceptually equal to 1 gram divided by Avogadros constant. Byjus Asked on April 5 2016 in Chemistry. The protons and neutrons of the nucleus account for nearly all of the total mass of atoms with the electrons and nuclear binding energy making minor.
Related words and phrases Measurements of weight mass. Amu A unit of mass equal to 112 the mass of the most abundant isotope of carbon carbon-12 which is assigned a mass of 12. The unit of measure for mass is the atomic mass unit amu.
Atomic Mass Unit AMU The periodic table of elements contains every atom known to mankind. Atomic mass indicates the size of an atom. AMU is used to differentiate between isotopes.
A unit of mass for expressing masses of atoms molecules or nuclear particles equal to ¹₁₂ the mass of a single atom of the most abundant carbon isotope 12C called also dalton Examples of atomic mass unit in a Sentence.
 Entiteta Gooey Smisel Atomic Mass Unit Susiessewingsensations Com
Entiteta Gooey Smisel Atomic Mass Unit Susiessewingsensations Com
 Define The Atomic Mass Unit Brainly In
Define The Atomic Mass Unit Brainly In
 Atomic Mass Unit Amu Definition Standard Conversion Video Lesson Transcript Study Com
Atomic Mass Unit Amu Definition Standard Conversion Video Lesson Transcript Study Com
 Entiteta Gooey Smisel Atomic Mass Unit Susiessewingsensations Com
Entiteta Gooey Smisel Atomic Mass Unit Susiessewingsensations Com
 Define The Atomic Mass Unit Youtube
Define The Atomic Mass Unit Youtube
 A Define Atomic Mass Unit What Is Its Symbol B Define Atomic Mass Of An Element C What Is Youtube
A Define Atomic Mass Unit What Is Its Symbol B Define Atomic Mass Of An Element C What Is Youtube
 Pin By Makaylah Randolph On Chemistry Education Atomic Mass Unit Chemistry Education Science Literacy
Pin By Makaylah Randolph On Chemistry Education Atomic Mass Unit Chemistry Education Science Literacy
 Define The Atomic Mass Unit Science Atoms And Molecules 13231495 Meritnation Com
Define The Atomic Mass Unit Science Atoms And Molecules 13231495 Meritnation Com