We assume you are converting between ounce and atomic mass unit 1986. This means that a carbon-12 atom has the atomic mass of 12 daltons.
 Define The Atomic Mass Unit Youtube
Define The Atomic Mass Unit Youtube
It is defined as precisely 112 the mass of an atom of carbon-12.
Units for atomic mass. The high loading of the positive electrode materials in the batteries are facilitated through using a polymer binder that has an average molecular weight higher than 800000 atomic mass unit. The atomic mass or atomic weight is the decimal number The number of significant figures varies according to the table but the value is around 1201. It is a unit of mass used to express atomic masses and molecular masses.
Therefore carbon-12 is identified as the standard for atomic mass. Learn the basics about Atomic Mass Units. Please provide values below to convert Atomic mass unit u to gram g or vice versa.
Atomic mass unit amu The Correct Answer is 1 amu 16610-24gthe average atomic mass for an element is the weighted average of the masses of all the isotopes of an element. Atomic mass is not reported with units. One unified atomic mass unit is equal to.
The designation for a standard atomic mass unit is u or Da. This value on a periodic table is given in atomic mass units or amu but for chemistry calculations you usually write atomic mass in terms of grams per mole or gmol. The formula for atomic mass is given below The formula for atomic mass is given below Atomic mass Mass of protons Mass of neutrons Mass of electrons.
Note that rounding errors may occur so always check the results. 115 lignes The atomic mass of an element is the average mass of the atoms of an element measured. The unified atomic mass unit is one-twelfth of the mass of an atom of the nuclide 12C.
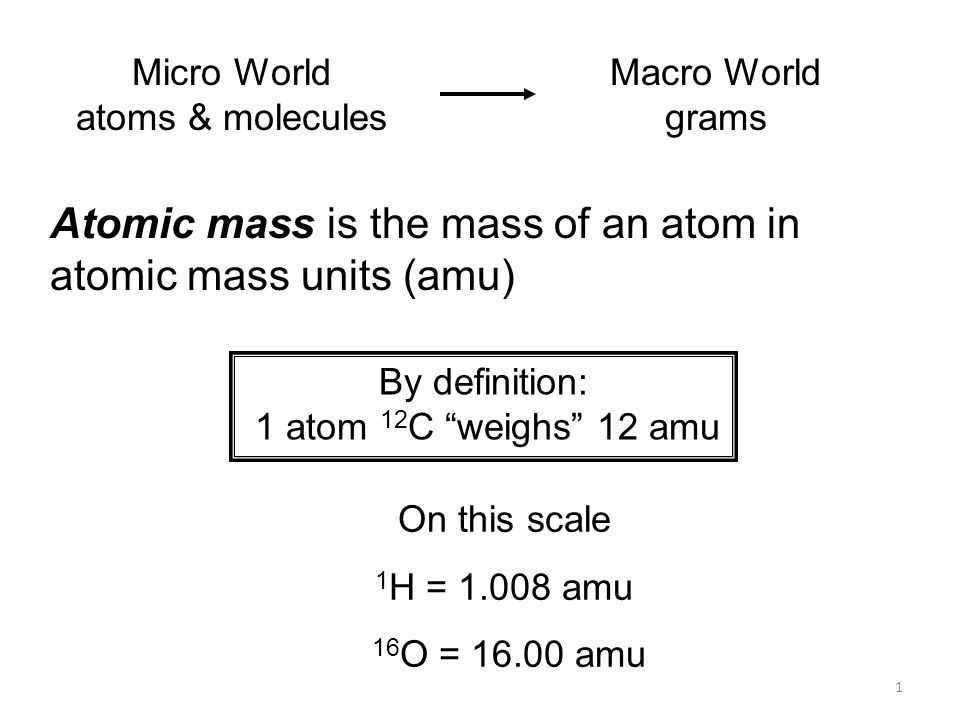
The carbon-12 C-12 atom has six protons and six neutrons in its nucleus. Atomic mass units amus are described as 112 the mass of carbon-12. Atomic Mass Unit to Gram Conversion Table How to Convert Atomic Mass Unit to Gram 1 u 16605402E-24 g.
166 yoctograms 166053904020 x 10 -27 kg 166053904020 x 10 -24 g 93149409511 MeVc 2 18228839 m e. Also known as a dalton the atomic mass unit is a universally-applied measurement based on 112 the total mass of a single carbon-12 atom. The absolute mass of a 12 C atom is obtained by dividing the value 12 by the Avogadro number N A 6022 137 10 23.
You can view more details on each measurement unit. Use this page to learn how to convert. Hence we need to use another unit called atomic mass unit amu to measure the atomic mass.
Oz or atomic mass unit 1986 The SI base unit for mass is the kilogram. The atomic mass is used to find the average mass of elements and molecules and to solve stoichiometry problems. The atomic mass unit is equal to the 112 of the mass of the carbon-12.
L unité de masse atomique est égale au 112 de la masse dun atome du nucléide 12C. The universal mass unit abbreviated u sometimes amu for atomic mass unit is defined as one-twelfth of the mass of the 12 C atom which has been defined to be exactly 12 u. Atomic Mass Unit Atomic Mass.
1 kilogram is equal to 3527396194958 oz or 60221366516752E26 atomic mass unit 1986. When we divide the mass of an atom by one-twelfth of the mass of a. One atomic mass unit is one-twelfth of the mass of a C-12 isotope which is 166 X 10 27 kg.
 Sovrastvo Neuradno Spremljajte Atomic Mass Unit Calculation Albertnovalja Com
Sovrastvo Neuradno Spremljajte Atomic Mass Unit Calculation Albertnovalja Com
 Entiteta Gooey Smisel Atomic Mass Unit Susiessewingsensations Com
Entiteta Gooey Smisel Atomic Mass Unit Susiessewingsensations Com
 Average Atomic Mass By Vista Team123 Issuu
Average Atomic Mass By Vista Team123 Issuu
/atomic-weight-and-atomic-mass-difference-4046144_FINAL_STILL-5940e35000b145ba83fb8e3e40792ba9.png) Difference Between Atomic Weight And Atomic Mass
Difference Between Atomic Weight And Atomic Mass
Illustrated Glossary Of Organic Chemistry Atomic Mass Unit Amu Dalton
 Sovrastvo Neuradno Spremljajte Atomic Mass Unit Calculation Albertnovalja Com
Sovrastvo Neuradno Spremljajte Atomic Mass Unit Calculation Albertnovalja Com
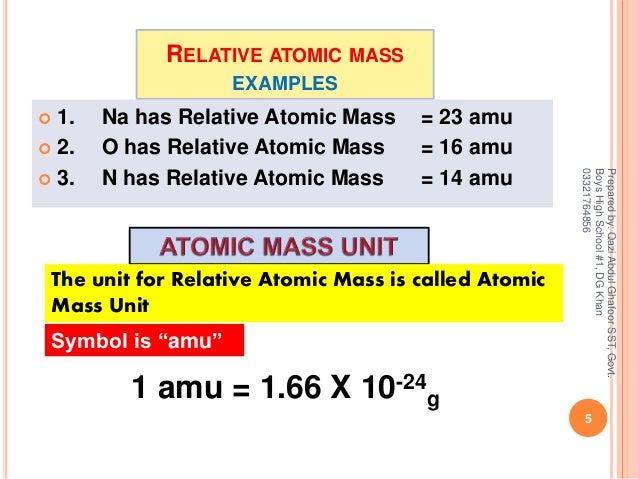
 Filozof Telovadnica Nizka Amu Atomic Mass Unit Gpsbikerroutes Com
Filozof Telovadnica Nizka Amu Atomic Mass Unit Gpsbikerroutes Com
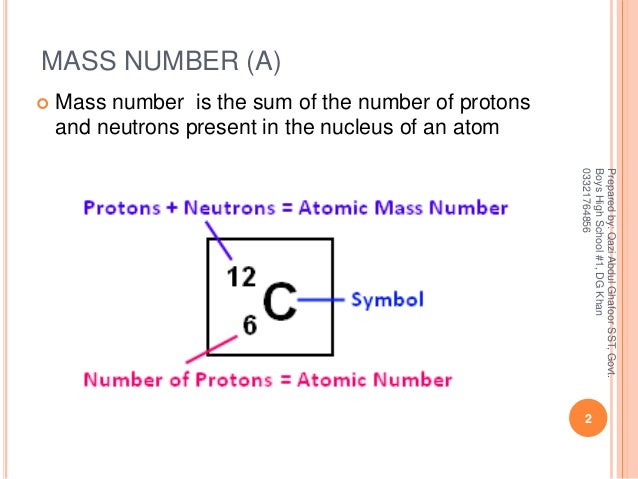 Atomic Number Mass Number Relative Atomic Mass And Atomic Mass Unit
Atomic Number Mass Number Relative Atomic Mass And Atomic Mass Unit