The boiling point is the temperature at which the vapor pressure is equal to the atmospheric pressure. Water boils at 100 degrees Celsius at 1 atm of pressure but a solution of saltwater does not.
 Physical Changes And Mixtures And Solutions Physical Change Physics Middle School Science
Physical Changes And Mixtures And Solutions Physical Change Physics Middle School Science
At 10000 feet above sea level the pressure of the atmosphere is only 526 mmHg.

Boiling point and freezing point of water. Boiling Point Calculator Water. So 100ºC is now the boiling point of water while 0ºC is the freezing point of water. On the melting point of ice.
For example the boiling point of pure water at 10 atm is 100 o C while the boiling point of a 2 saltwater solution is about 102 o C. For water it is 0512The boiling point of water increases by 17 CStep 3. This effect is known as that of a freezing mixture.
Impurities in the water such as sea salt pressure and altitude will raise or lower the freezing point. Salt water consequently boils at a temperature above 100 0 C. Under normal conditions when the pressure of the atmosphere is approximately 760 mmHg water boils at 100 o C.
Since evaporation is suppressed the boiling point is raised. In theory the two temperatures would be the same but liquids can be supercooled beyond their freezing points so that they dont solidify until well below freezing point. Pure water has a freezing point of 32 o F 0 o C at sea level under normal pressure.
The melting point for water is 0 degrees C 32 degrees F. Enter the freezing point depression constant and the molality into the calculator to determine the freezing point of the liquid. The red line shows the atmospheric pressure of the selected planet.
Some of the solute salt molecules occupy the surface reducing the effective area for evaporation. The boiling point elevation is the amount that the boiling point temperature increases compared to the original solvent. In terms of vapour pressure pressure exerted by the vapours of solution Boiling point of a liquid is the temperature at which vapour pressure of the liquid becomes equal.
When the opposite happens and a liquid turns into a solid it is called freezing. When a liquid becomes a gas it is called boiling. When a salt is added to the water some of the salt molecules occupy the space near the surface of the liquid.
Find freezing point depressionWhen a non volatile solute is added to water it results in decrease in freezing point. BOILING FREEZING POINTS Pure water as you may know has a boiling point of 212F 100C and a freezing point of 32F 0C. Δ T b K b.
Textatm is 100texto textC while the boiling point of a 2. However when you create a 5050 mixture using water and ethylene glycol the boiling point rises to 223F 106C and the freezing point lowers to -35F -37C. The change in the boiling point is calculated from.
Originally Anders Celsius assigned zero to stand for waters boiling point and 100 to stand for waters freezing point. A solution boils at a slightly higher temperature than the pure solvent. Because the freezing point of pure water is 0C the sucrose solution freezes at 068C.
For example the boiling point of pure water at 10. The freezing point depression is the amount that the freezing temperature decreases. The formula to find freezing point depression is Kf is constant for water and its value is 186The freezing point of water decreases by 62 CTherefore we can say that the solution will have a.
What temperature does water boil at 10000. The freezing point describes the liquid to solid transition while the melting point is the temperature at which water goes from a solid ice to liquid water. A similar property of solutions is boiling point elevation.
The boiling point of water is 1000C or 373 K. This was later reversed by Carolus Linnaeus or Carl Linnaeus a Swedish botanist physician and zoologist after Anders died. For pure water the boiling point is 100 degrees Celsius 212 Fahrenheit at one atmosphere of pressure and the melting point is 0 degrees Celsius 32 degrees Fahrenheit at one atmosphere of pressure.
The Celsius scale sets the freezing point and boiling point of water at 0C and 100C respectively. A mathematical equation is used to calculate the boiling point elevation or the freezing point depression. About Press Copyright Contact us Creators Advertise Developers Terms Privacy Policy Safety How YouTube works Test new features Press Copyright Contact us Creators.
When you take it one step further. The normal boiling point of water is 100 o C because this is the temperature at which the vapor pressure of water is 760 mmHg or 1 atm. This Demonstration shows the boiling temperature of water on six planets all of which have different atmospheric pressures.
Also to know is what is boiling and freezing point. The Fahrenheit scale defines the freezing point of water as 32F and the boiling point as 212F. At at high altitudes the lower pressure makes the boiling point several degrees lower.
Therefore the boiling point elevation would be 2 o C.
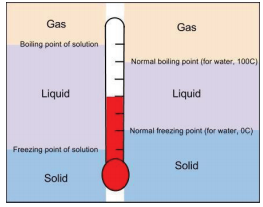 13 9 Freezing Point Depression And Boiling Point Elevation Making Water Freeze Colder And Boil Hotter Chemistry Libretexts
13 9 Freezing Point Depression And Boiling Point Elevation Making Water Freeze Colder And Boil Hotter Chemistry Libretexts
/the-freezing-point-of-water-609418_FINAL-01f50f5f4f7d4a39854bebcc59df1aa4.gif) What Is The Freezing Point Of Water
What Is The Freezing Point Of Water
 Boiling Point Freezing Melting Point Anchorchart Teaching Science Science Lessons Science Anchor Charts
Boiling Point Freezing Melting Point Anchorchart Teaching Science Science Lessons Science Anchor Charts
/the-freezing-point-of-water-609418_FINAL-01f50f5f4f7d4a39854bebcc59df1aa4.gif) What Is The Freezing Point Of Water
What Is The Freezing Point Of Water
 What Is Water S Boiling Point In Fahrenheit The Millennial Mirror
What Is Water S Boiling Point In Fahrenheit The Millennial Mirror
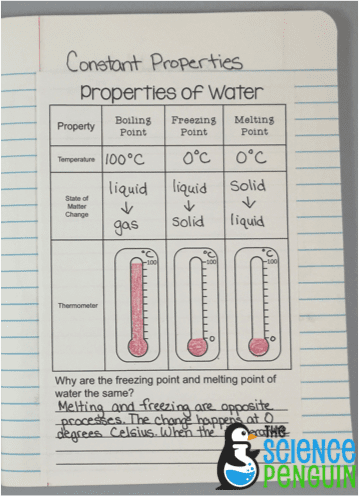 Constant Properties Of Water Boiling Point Melting Point And Freezing Point The Science Penguin
Constant Properties Of Water Boiling Point Melting Point And Freezing Point The Science Penguin