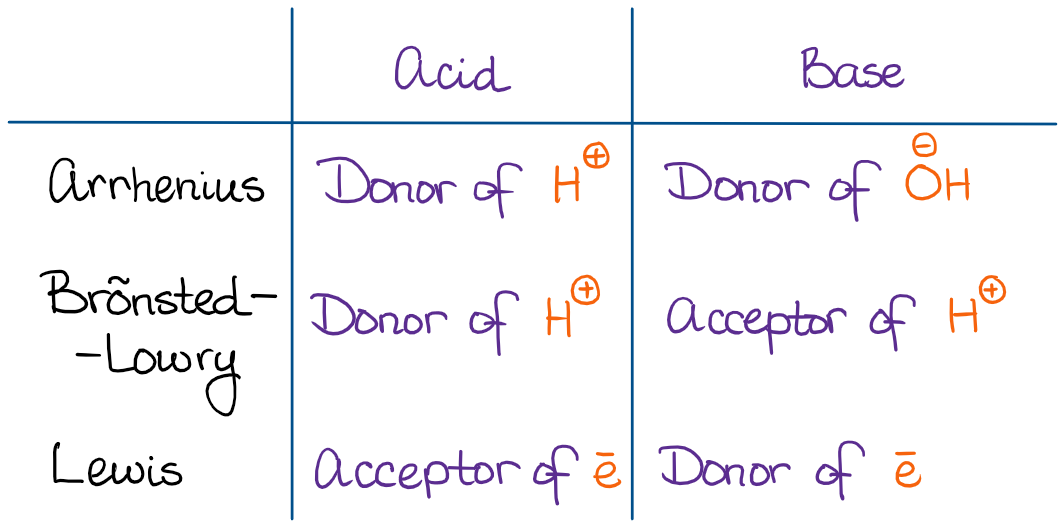
L the Water or Arrhenius Theory. This Module describes the Arrhenius Brønsted-Lowry and Lewis theories of acids and bases and explains the relationships between them.
Modern Theories Of Acids Bases The Arrhenius And
The Arrhenius theory According to Arrhenius theory.

Theories of acids and bases. A base is a substance which ionises in water to give hydroxide ions OH. A base is a proton hydrogen ion acceptor. The Arrhenius Theory of acids and bases.
There are three concepts of acids and bases in current use. It also explains the concept of a conjugate pair - an acid and its conjugate base or a base and its conjugate acid. A pair of species differing by a single proton is called a conjugate acidbase pair.
This we can conclude because it has a lot more specific information that is more developed than that of Arrheniuss. An acid is a proton hydrogen ion donor. Oxygene means acid-forming in Greek and reflects the mistaken belief that the element oxygen was responsible for a compounds acidic properties.
Guidance Students should know the representation of a proton in aqueous solution as both H aq and H 3 O aq. A BrønstedLowry acid is a protonH donor and a BrønstedLowry base is a protonH acceptor. They are Arrhenius Theory.
So which theory Arrhenius or Bronsted-Lowry is the most suitable to the society then. Amphiprotic species can act as both BrønstedLowry acids and bases. Arrhenius Theory The Swedish chemist Svante Arrhenius published his theory of acids and bases in 1887.
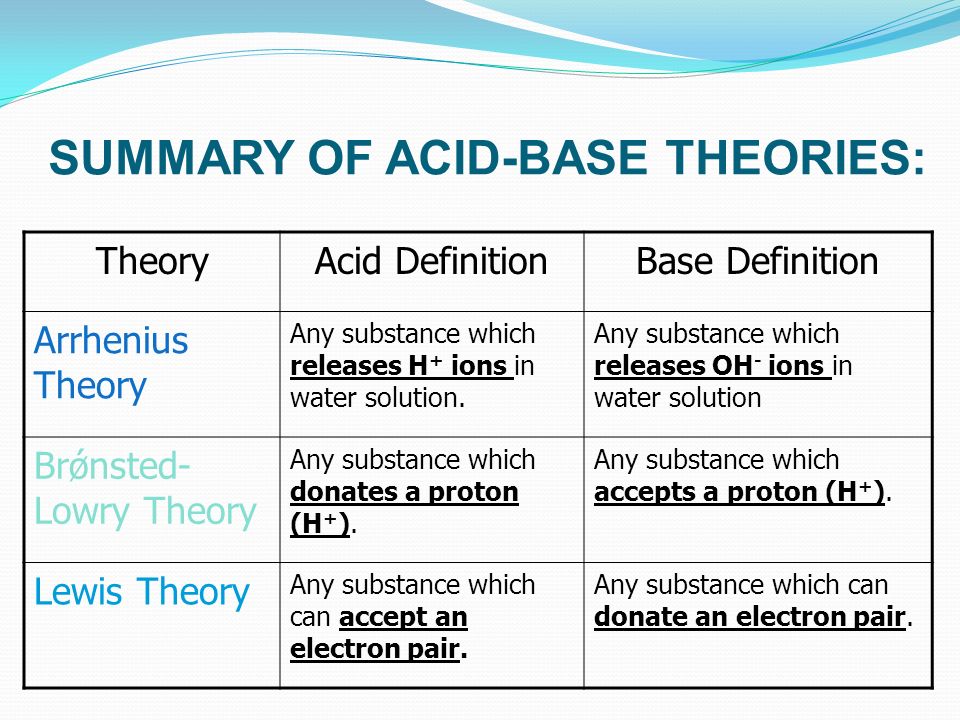
Neutralisation happens because hydrogen ions and hydroxide ions react to produce water. According to Lewis theory of acids and bases it states that acids as electron pair acceptors and bases as an electron pair donors. These theories include the Arrhenius theory the Bronsted-Lowry theory and the Lewis theory of acids and bases.
This page just tidies things up. Remember however that the aqueous hydrogen ion is actually chemically bonded to water that is H3O. Acid can remain energetically favorable after a loss of H molecule.
Hydrochloric acid is neutralised by both sodium hydroxide. Limitations of the theory. Acidic substances are usually sour in nature and are primarily a molecule that has the capacity to donate an H ion.
Much more accurate definitions of acids and bases have been created. The theory of acids and bases like many other chemical theories has undergone numerous changes in recent times. Arrhenius Concept of Acids and Bases According to the Arrhenius concept of acids and bases an acid is a substance that when dissolved in water increases the concentration of hydronium ion H3O.
It also explains the concept of a conjugate pair - an acid and its conjugate base or a base and its conjugate acid. The Bronsted-Lowry Theory of acids and bases. If you have worked through the various reactions of acids and bases in this section then you will have met almost all of the reactions you need to know about.
Three concepts are explained this acids bases mechanism. An acid is a substance which ionises in water to give hydrogen ions H. 2 the Proton or Br0nsted-Lowrv Theory.
After made researches we can conclude that Bronsted-Lowrys theory on acids and bases is the most suitable theory to the society. An acid is a substance which dissociates in water to produce one or more hydrogen ions H. Acids are substances which produce hydrogen ions in solution.
Three different theories have been put forth in order to define acids and bases. The Arrhenius theory wouldnt count this as an acid-base reaction despite the fact that it is producing the same product as when the two substances were in solution. An acid is a hydrogen ion.
Acid-base theory has been developed by scientists around the world and its vocabulary has been influenced by their languages. Always the changes have been such as to make the theory more general. What is the Bronsted-Lowry theory.
The three main theories in use today are. THEORIES OF ACIDS AND BASES This page describes the Arrhenius Bronsted-Lowry and Lewis theories of acids and bases and explains the relationships between them. There are three different theories of what acids and bases are but the most useful one at this level is the Bronsted-Lowry theory.
A brief description of each of these theories is provided in this subsection. Acids and bases can be defined via three different theories. Bases are substances which produce hydroxide ions in solution.
Theories of Acids and Bases. It can be simply explained by these two points. Arrhenius Acids and Bases 1.
Strong and Weak Acids. They are based on the structure and composition of acids bases. Theories of acids and bases 8.
 Historical Development Of Acid Base Theories Ms Jonasson S Classes
Historical Development Of Acid Base Theories Ms Jonasson S Classes
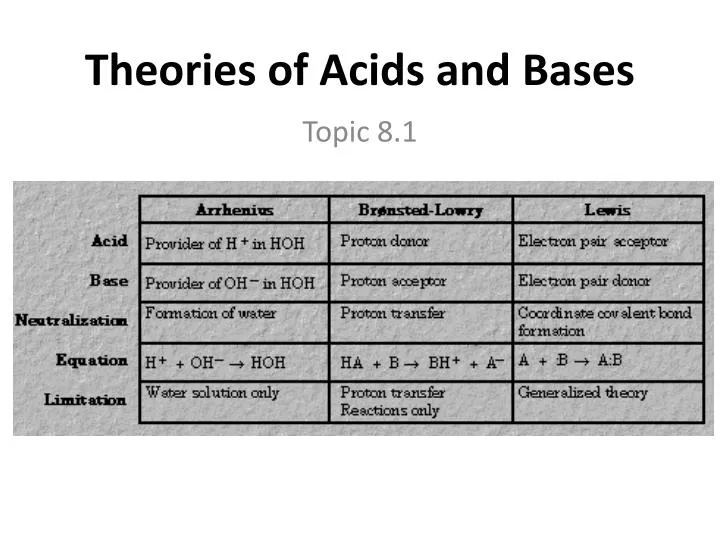 Ppt Theories Of Acids And Bases Powerpoint Presentation Free Download Id 6263508
Ppt Theories Of Acids And Bases Powerpoint Presentation Free Download Id 6263508
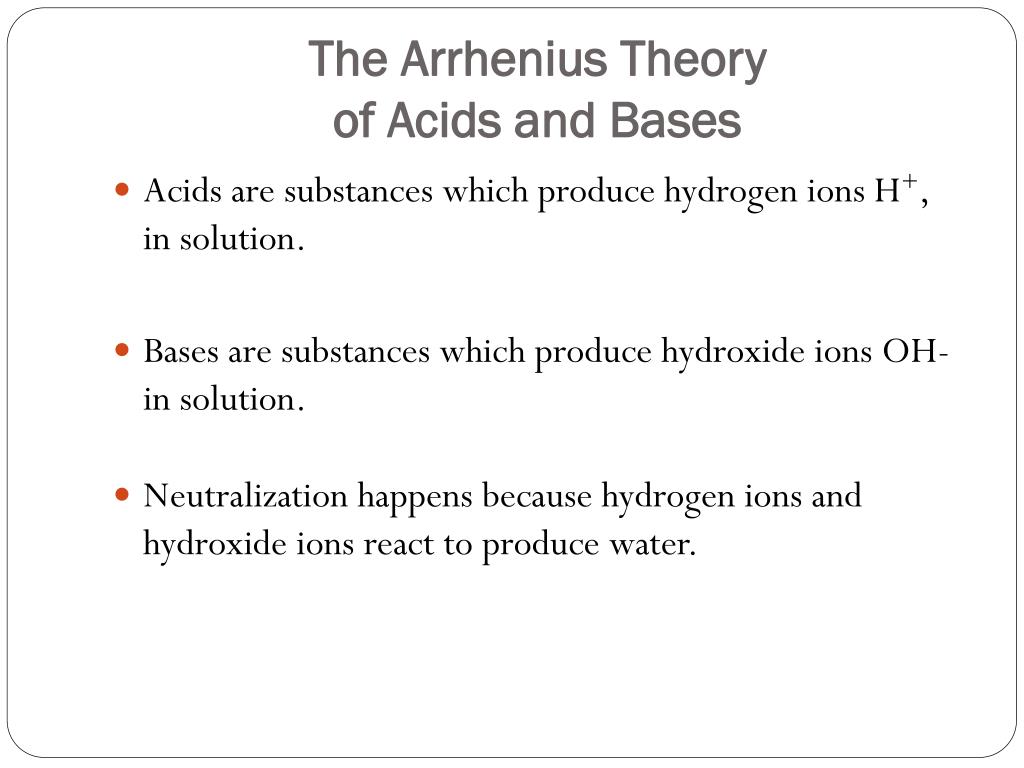 Ppt The Arrhenius Theory Of Acids And Bases Powerpoint Presentation Free Download Id 3092912
Ppt The Arrhenius Theory Of Acids And Bases Powerpoint Presentation Free Download Id 3092912
 Introduction To Acids And Bases In Organic Chemistry Organic Chemistry Tutor
Introduction To Acids And Bases In Organic Chemistry Organic Chemistry Tutor
 1 Theories Of Acids And Bases A Arrhenius Theory Chegg Com
1 Theories Of Acids And Bases A Arrhenius Theory Chegg Com
 Acids Bases Salts The Arrhenius Theory Of Acids And Bases Ppt Download
Acids Bases Salts The Arrhenius Theory Of Acids And Bases Ppt Download
 Chapter 15 Acids And Bases 2 Acid Base Theories In Defining What Is Considered To Be An Acid And What Is Considered To Be A Base Three Theories Have Ppt Download
Chapter 15 Acids And Bases 2 Acid Base Theories In Defining What Is Considered To Be An Acid And What Is Considered To Be A Base Three Theories Have Ppt Download